Chemical bonding – Page 3
-
 Research
ResearchSimulation says supercritical water has no hydrogen bonds
Computational approach seeks to clarify bonding confusion
-
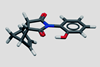 Research
ResearchHydrogen bond imparts more stability on transition state than expected
Study reveals importance of repulsive interactions
-
 Research
ResearchMacrocycles slide freely across carbon nanotubes
Simulations suggest macrocycles show superlubricity at room temperature
-
 Research
ResearchExcited state potential energy curves reignite diatomic carbon’s bond order conundrum
Molecular orbital theory-based approach backs quadruple bond
-
 Research
ResearchBeautiful molecules ring up with a purpose
How Stephen Goldup used his experience in organic synthesis to find a supramolecular niche
-
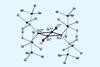 Research
ResearchNew silicon–silicon bond is a rare example of a π bond without a σ bond
Discovery made in silicon analogue of bicyclobutane
-
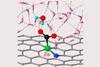 Research
ResearchHydrogen bonds are single atom nickel’s secret for carbon dioxide reduction
Computational calculations show that hydrogen atoms and charge capacity are behind nickel’s high activity and selectivity in electrochemical carbon dioxide reduction
-
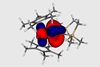 Research
ResearchNew type of bond is the first to defy actinide contraction trend
Bonds discovered in metallacycles are unique to the 5f elements
-
![An image showing a [2]catenane](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/100x67/0/0/4/504004_fja0c01757_0007.jpeg_49119.jpg) Research
ResearchMechanical protecting group shields molecules from stress and strain
Catenane makes force-activated molecules more resistant mechanical bond breaking
-
![An image showing the bonding pattern of [2]catenane](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/100x67/2/8/7/503287_c9cc09483kf2_286914.png) Research
ResearchAromaticity lends stability to carbon-only catenanes
Catenanes of newly discovered C18 rings are theoretically possible thanks to the kinetic quirks of aromaticity
-
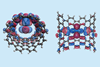 Research
ResearchRhenium plays starring role in bond movie
Controllable energy input from transmission electron microscope captures atoms dissociating and recombining
-

-
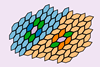 Research
ResearchOdd 'entropic bonds' akin to chemical ones can form between nanoparticles
Simulations finds that ’bonding’ can occur in the absence of any electronic interaction
-
 Opinion
OpinionWill computers ever discover drugs from scratch?
With enough understanding and computing power, it should be possible, but will it happen?
-
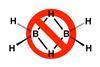 Research
ResearchThree centres, two electrons, but how many bonds?
Tweaking Lewis structures allows for a simplified and clearer representation of electron-deficient molecules
-
 Research
ResearchCarbynes expand the possibilities of carbon–carbon double bonds
Chemists use novel monovalent carbon species to insert functionalities in the middle of double bonds
-
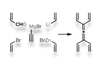
 Research
ResearchGiant tree molecule made out of 45 rotaxanes switches size on demand
Mechanically interlocked molecules give dendrimer the ability to expand and contract
-
 Research
ResearchFirst ever synthesis of most double-bond-rich hydrocarbon
Tiny hydrocarbon with six double bonds has unrivalled ability to make complex ring systems though multiple Diels–Alder reactions
-

-
 Research
ResearchFirst molecular knots made from just benzene rings
Catenane and trefoil knot are the first mechanically interlocked molecules made from only carbon and hydrogen