New type of bond is the first to defy actinide contraction trend
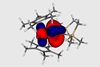
Bonds discovered in metallacycles are unique to the 5f elements
Protactinium, uranium, neptunium and plutonium are defying trends with new interactions: head-to-side δ and side-to-side φ bonds. These bonds, chemists have discovered, are part of the reason actinide metallacycles don’t follow the contraction trend – the decrease in metal–ligand bond lengths in heavier elements.
