Hydrogen bonds are single atom nickel’s secret for carbon dioxide reduction
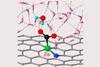
Computational calculations show that hydrogen atoms and charge capacity are behind nickel’s high activity and selectivity in electrochemical carbon dioxide reduction
Hydrogen bonds and charge capacity are the secret to nickel atoms’ outstanding ability to reduce carbon dioxide, researchers have discovered. Understanding the reaction mechanism better could lead to more efficient catalysts for turning carbon into useful chemicals – like fuels or materials – to reduce our dependence on the fossil fuels that generate the climate change gas in the first place.
