When does a hydrogen bond become a covalent bond?
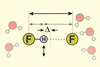
Ultrafast infrared spectroscopy probes the character of the short, strong bonds in HF2–
In May 1919, an undergraduate chemistry student named Maurice Huggins at the University of California at Berkeley was panicking. His professor William Bray required his students to write a term paper to pass his course, but Huggins hadn’t yet done that, and the deadline was approaching. In desperation, he showed Bray rough notes he’d made about some of the ‘unsolved problems in chemistry’ that Bray had discussed. Might the professor accept these notes as the term paper, with a title added?
Bray did, but with caveats. ‘Huggins,’ he said, ‘there are several interesting ideas in this paper, but there is one you’ll never get chemists to believe: the idea that a hydrogen atom can be bonded to two other atoms at the same time.’ Huggins had advanced this strange idea using the electron-sharing theory of chemical bonding proposed by Gilbert Lewis, depicting, for example, a dimer of hydrogen fluoride in which the four atoms were arranged in a square, with each hydrogen bonded to both fluorines. Lewis, however, was sceptical of the whole notion.