First inspection of σ-hole reveals its peculiar shape
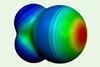
Atomic force microscopy snaps first experimental images of halogen bond’s charge distribution
The first experimental images of a bromine atom’s σ-hole have been unveiled, mapping the peculiar charge distribution that is the basis for halogen bonding.
Although it seems counterintuitive that two negatively charged atoms attract rather than repel each other, this is the case when it comes to halogen bonding. Halogen atoms form attractive intermolecular bonds with other halogens, as well as electron donors such as oxygen. Halogen bonds – variations of which also exist in elements of other groups – are essential for holding together supramolecular crystals and even biological macromolecular structures.