Hydrogen-free hydrogenation offers simple solution for medicinal chemists
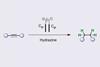
New method expands electrochemical hydrogenation’s scope
An electrochemical hydrogenation reaction could be a practical new tool for laboratory chemists. The technique eliminates the need for the hydrogen gas and expensive transition metal catalysts that are typically used in hydrogenations. It is also tolerant of many different functional groups, making it well suited for late-stage chemistry.
Hydrogenation reactions are a mainstay of synthetic chemistry, but they usually require catalysts based on rare and expensive metals like ruthenium, rhodium or iridium. These reactions also tend to use harsh reaction conditions, including high pressures of hydrogen gas. Over the last decade electrochemical alternatives have been developed, but these have faced challenges, in particular the limited scope of substrates that can be used.
