Clever ligand design enables alcohol-directed C-H activation
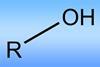
Despite being the most common functional group in nature alcohols have proven difficult to functionalise
Designer ligands have promoted what the team behind the work believes is the first alcohol-directed activation of unreactive sp3 C-H bonds. Incorporating hydrogen-bonding interactions into the design strengthened and organised the reactive complex, an approach which could be applied to other catalytic systems.
C-H functionalisation is one of the most versatile reactions in organic synthesis but the ubiquity and inertness of these bonds make selective transformations incredibly challenging. Directed C-H activation attempts to overcome this by employing existing functionality within the substrate to anchor the metal catalyst close to a particular bond and carefully designed ligands allow chemists to use carbonyl and nitrogen-containing groups to direct the initial metal association interaction.
