Skeletal editing that simply swaps aromatic carbons for nitrogens will aid drug discovery
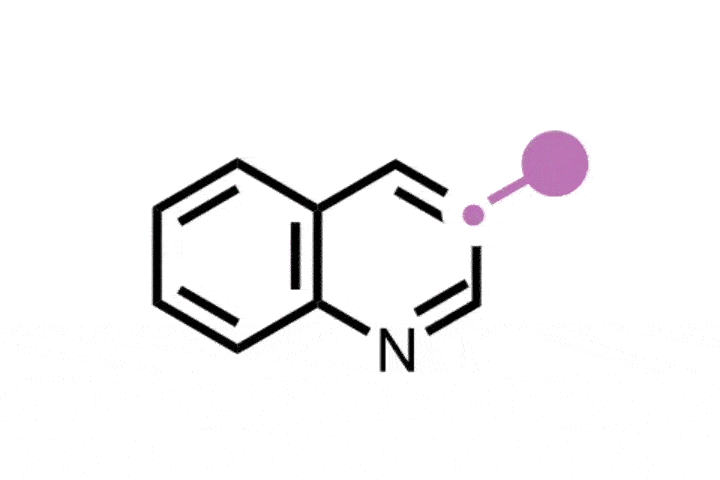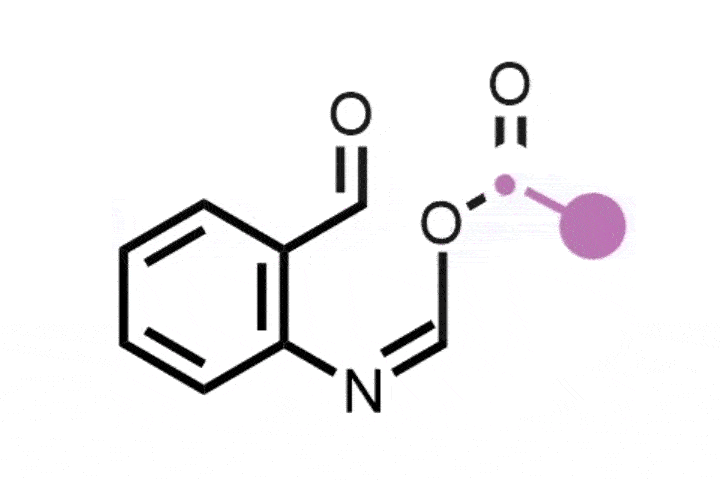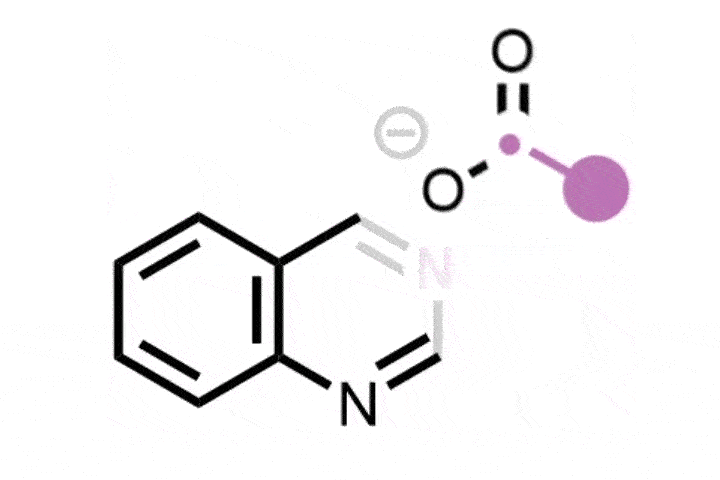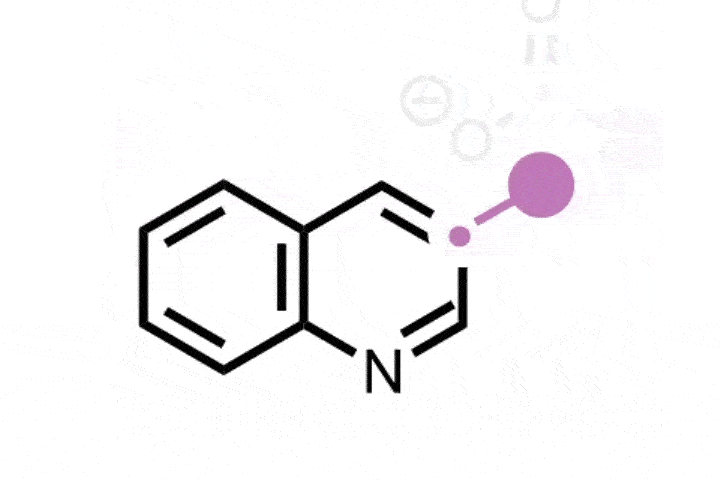
Atom-swapping chemistry gains two new techniques that are ready to use
Two new reactions enable synthetic chemists to easily swap aromatic carbon atoms for nitrogen atoms, allowing the quick conversion of aromatic groups into heteroaromatics. The reactions will help drug discovery chemists quickly modify chemical structures to see how small structural variations influence the biological activity of compounds of interest. The researchers behind the work believe it will allow chemists to synthesise chemicals in a more intuitive way that closely matches the way the researchers actually conceptualise their target molecules.
The field of ‘skeletal editing’ is a fast developing area of synthetic chemistry, with many researchers devising new techniques to alter the structures of complex molecules in a variety of ways – whether by adding, deleting or swapping single atoms in a compound’s core motif. Mark Levin’s group at the University of Chicago, US, is particularly interested in editing techniques involving nitrogen atoms. In 2021, the group developed a method for deleting nitrogen atoms from organic structures, and last year the team revealed methods for deleting carbons from nitrogen containing aromatic groups and inserting nitrides into indenes to generate isoquinolines.
