All stereochemistry articles
-
 Research
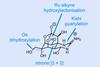
ResearchChirality-flipping reaction could completely change total synthesis strategies
Photocatalytic reaction that inverts configuration of chiral carbon centres offers new stereochemical editing logic
-
-
-
 Research
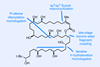
ResearchAutomated carbon-13 NMR structure validation program highlights errors atom-by-atom
Introducing a new measure to quantify molecular structural uncertainty
-
 Webinar
WebinarResolving absolute stereochemistry in early drug discovery with VCD
From sample preparation to use of quantum chemical software tools, learn how vibrational circular dichroism (VCD) streamlines chiral analysis in the R&D analytical support lab
-
-
 Research
ResearchMachine-learning tool performs stereochemical assignments on SPM images
Identifying chiral centres on SPM images with machine-learning tools only takes a few hours and could save researchers time
-
 Research
ResearchNewly discovered marine compound is in a super-carbon-chain league of its own
Isolated from algae, Benthol A has 35 stereocentres
-
 Article
ArticleChemistry pedagogy for a modern world
The Royal Society of Chemistry has taken a new approach to teaching higher education students the fundamentals of maths and stereochemistry, putting students themselves at the heart of the process
-
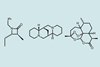 Research
ResearchProgram that automatically interprets NMR spectra is boon for structure elucidation
Raw NMR data takes 60 seconds, rather than eight hours, to go from spectrometer to structure
-
-
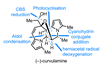
 Research
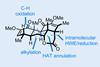
ResearchSynthetic strategy exploits fluxional nitrogen to deliver three chiral centres for the price of one
Innovative method amplifies chirality and complexity in medicinally relevant cyclic hydrazines
-
 Research
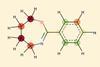
Research‘Inside-out’ chirality discovered by chance could have been missed in many molecules
A structure assignment odyssey finds a natural product with a type of isomerism never before seen in small molecules
-
 Research
ResearchExplosive 25% more powerful than TNT found in first study of energetic isomers
Stereo- and regiochemistry affect compounds’ explosive potential
-
 Research
ResearchChiral borane complexes catalyse new synthesis opportunities
N-heterocyclic carbene-borane complexes with a rare stereogenic boron centre demonstrate potential in stereoselective catalysis
-
 Research
ResearchFlexible catalyst systems open up nitrile chemistry
One new strategy enantioselectively produces amines from nitriles, and another generates valuable alkenyl nitriles
-
 Research
ResearchWalking radicals relocate double bonds
Nickel-guided radicals walk along carbon chains to make E-alkenes from terminal alkenes
-
 Research
ResearchFlexible route to enantiomerically enriched cyclobutanes
System for modifying square starting molecule could be gateway to numerous bioactive molecules
-
 Research
ResearchRound-the-ring catalysis makes cyclic peptides chiral
Classic hydrogenation catalyst installs multiple stereocentres one by one to create small amino acid rings as single enantiomers
-
 Research
ResearchFirst new form of isomerism discovered in 50 years will be the last
Porphyrin–boron compounds have revealed a curious form of molecular chirality based on hindered bond angle inversion