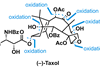
(–)-Taxol
Following nature’s lead
For all the talk of biological activity in the introductions of total synthesis papers, it’s pretty rare that the Venn diagram circles of ‘molecules useful to society’ and ‘molecules synthetic chemists want to make’ coincide. That’s not because natural products are bad drugs; indeed, between 1981 and 2014, over a quarter of all FDA-approved drugs were natural products or their derivatives. However, for a natural product to be a viable medicine it usually needs to be easily available from natural sources, which rather takes the fun out of making it. But there have been exceptions.
As a blockbuster drug, Taxol (paclitaxel) is hardly scarce now, but it was quite hard to come by when it entered the clinic in the early 1990s. Back then, it was at the very limit of what chemists thought they could make in the lab. Combined with its limited natural supply, this made it a very popular target. At the height of Taxolmania, at least 30 groups were competing for the glory of being the first to prepare it, culminating in a photo finish between Robert Holton and KC Nicolaou’s groups in 1993–94. Now, 10 syntheses of Taxol have been reported and these days the molecule is more often seen on the pages of textbooks than journals.