Euonyminol
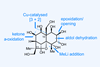
Overcoming the challenges of working with highly polar molecules
By this point of the year, a lot of people have broken their New Year’s resolutions. Not me, though. Since I resolved to cut out carbs, my life has never been better. You see, I’ve spent most of my full-time job for the past five years synthesising unnatural sugars, and this year I’m going to try and avoid carbohydrates for a while – at least while I’m in a lab coat.
Carbohydrates, by definition, contain rather a lot of oxygen, primarily in the form of hydroxyl groups. Undergraduate chemists learn early on that as the amount of oxygen (and nitrogen) in a molecule increases, so does its polarity. While non-polar compounds have certain challenges associated with them, these problems generally pale in comparison to those of extremely polar compounds. As polarity ramps up, purification starts to get tricky. Then your compounds stop coming out of water. At the extreme end of the spectrum, molecules start to get unexpectedly ‘sticky’. A colleague of mine recently worked with an intermediate that bound palladium so tightly that catalysis was impossible with less than stoichiometric metal. Even worse, the popular natural product ouabagenin, a hexahydroxy steroid, can famously pull the borate out of borosilicate glass!